Dr. Lei Chen's Group Revealed the Structure of a Pancreatic ATP-Sensitive Potassium Channel
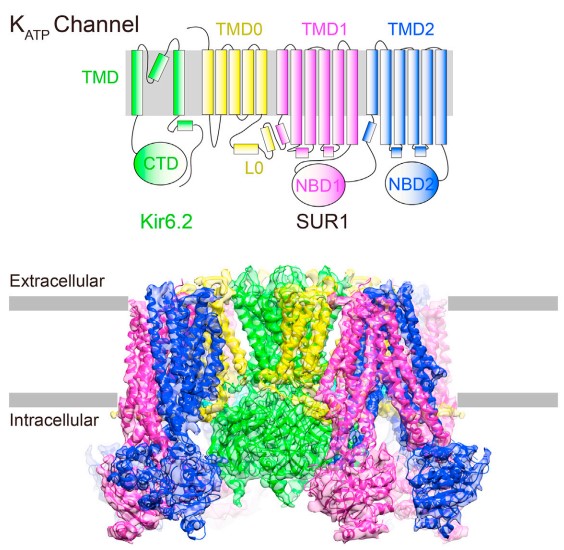
JANUARY 12, 2017: Lei Chen's group at the Peking-Tsinghua Center for Life Sciences recently published a paper on Cell (168, 101-110) titled 'Structure of a Pancreatic ATP-Sensitive Potassium Channel' with their collaborators, where they elucidated the pancreatic KATP channel structure at 5.6-Å resolution by cryo-EM.
Highlights
Pancreatic KATP channel structure is determined at 5.6-Å resolution by cryo-EM
TMD0-L0 fragment mediates the interactions between Kir6.2 and SUR1
An allosteric inhibitor bound to SUR1 stabilizes Kir6.2 in a closed conformation
PIP2 uncouples Kir6.2 from glibenclamide-bound SUR1
Summary
ATP-sensitive potassium channels (KATP) couple intracellular ATP levels with membrane excitability. These channels play crucial roles in many essential physiological processes and have been implicated extensively in a spectrum of metabolic diseases and disorders. To gain insight into the mechanism of KATP, we elucidated the structure of a hetero-octameric pancreatic KATP channel in complex with a non-competitive inhibitor glibenclamide by single-particle cryoelectron microscopy to 5.6-Å resolution. The structure shows that four SUR1 regulatory subunits locate peripherally and dock onto the central Kir6.2 channel tetramer through the SUR1 TMD0-L0 fragment. Glibenclamide-bound SUR1 uses TMD0-L0 fragment to stabilize Kir6.2 channel in a closed conformation. In another structural population, a putative co-purified phosphatidylinositol 4,5-bisphosphate (PIP2) molecule uncouples Kir6.2 from glibenclamide-bound SUR1. These structural observations suggest a molecular mechanism for KATP regulation by anti-diabetic sulfonylurea drugs, intracellular adenosine nucleotide concentrations, and PIP2 lipid.
Link for full-text article: http://www.cell.com/cell/fulltext/S0092-8674(16)31744-5

